“`html
Liquid Dietary Supplements Market: Growth, Trends & Qalitex’s Role in Ensuring Quality and Compliance
The Liquid Dietary Supplements Market is witnessing rapid expansion due to increasing consumer demand for convenient, efficacious, and bioavailable supplement formats. As the market grows globally, brands face mounting regulatory and quality assurance challenges. To maintain consumer safety, meet FDA requirements, and secure marketplace trust—especially on platforms like Amazon—these companies depend on specialized analytical and microbiological testing.
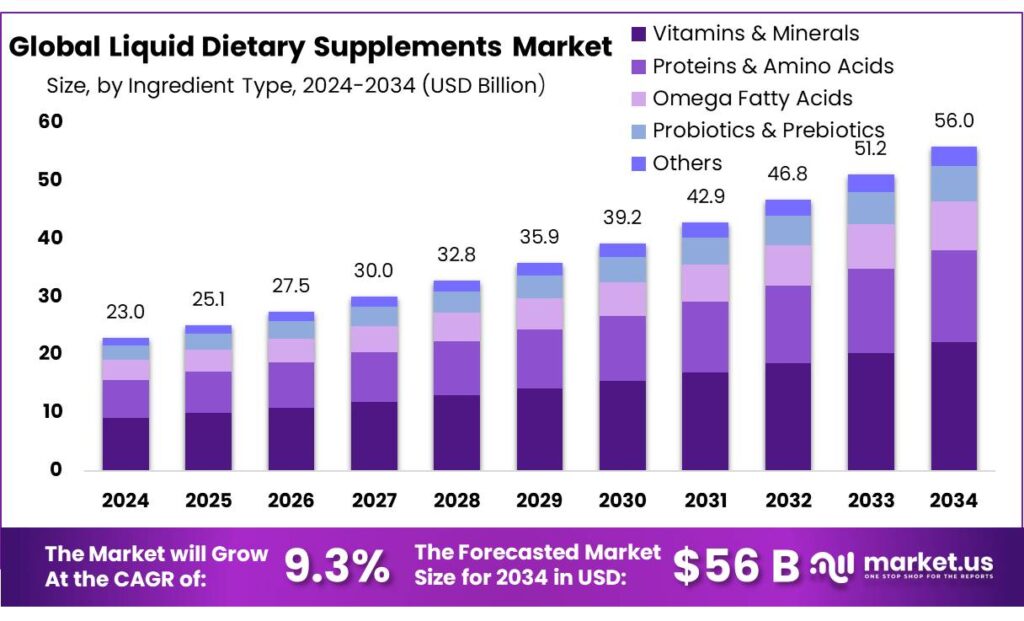
Market.us reports that the global liquid dietary supplements sector is projected to grow aggressively, driven by health-conscious consumers and rising awareness of functional nutrition. With the complexity of formulations increasing and strict regulatory frameworks evolving, choosing a reliable, accredited testing laboratory becomes essential for supplement manufacturers aiming to capture market share effectively.
Why Accreditation and Compliance Matter in Liquid Dietary Supplements Testing
Compliance with regulatory standards such as FDA guidelines, USP, and AOAC validated methods is imperative for liquid dietary supplements. Here’s why:
- ISO 17025 Accredited Testing Laboratory: Laboratories accredited with ISO/IEC 17025 demonstrate technical competence and reliable testing results that supplement brands can confidently use for product release and regulatory filings. Qalitex stands out as an ISO 17025 accredited testing laboratory based in Irvine, California, providing this level of quality assurance.
- FDA Compliant Product Testing: Ensuring products meet FDA safety and labeling regulations reduces risks of recalls and sanctions. Qalitex offers comprehensive FDA compliant product testing that addresses chemical, microbiological, and heavy metal limits specific to dietary supplements.
- USP & AOAC Validated Methods: These method validations are critical for consistency and accuracy in testing active ingredients and contaminants. Qalitex utilizes USP AOAC validated methods to support clients’ regulatory submissions and quality expectations.
- Regulatory Compliance Lab Services & Certificate of Analysis (CoA) Testing: A CoA signals trustworthy, batch-specific product data. Qalitex’s regulatory compliance lab services and certificate of analysis (CoA) testing empower brands to demonstrate compliance to retailers, regulators, and consumers.
Core Lab Services for the Liquid Dietary Supplements Market
Beyond accreditation, the depth of laboratory testing capabilities defines a testing partner’s value. Qalitex offers comprehensive core services designed specifically for liquid supplement manufacturers:
- Microbiology Testing Services: Detect microbial contamination risks in liquid formulations with precise microbiology testing services ensuring product safety and shelf integrity.
- Analytical Chemistry Lab Testing: Quantify active ingredients, excipients, and potential contaminants through sensitive chemical analyses. Qalitex provides industry-leading analytical chemistry lab testing to support formulation validation and label claims.
- Heavy Metal Analysis for Supplements: Heavy metals such as lead, arsenic, mercury, and cadmium pose safety threats. Qalitex conducts rigorous heavy metal analysis for supplements to detect and quantify impurities well within regulatory limits.
- Shelf-Life and Stability Studies: Stability protocols are essential to guarantee product efficacy until expiration. Qalitex’s shelf-life and stability studies help brands establish scientifically-backed expiry dates and proper storage conditions.
- Method Development and Validation Lab: When new liquid supplement formulations are developed, customized validated testing methods are critical. Qalitex’s method development and validation lab delivers tailored solutions ensuring accuracy, reproducibility, and compliance for innovative products.
Qalitex: Your One-Stop Quality-Control Partner in Southern California
Located in Irvine, California, Qalitex is uniquely positioned to serve Southern California’s booming dietary supplement manufacturers with fast turnaround times, scientifically rigorous testing, and regulatory insight. Whether you’re launching a new liquid dietary supplement, seeking Amazon-compliant testing, or requiring ongoing stability and CoA verification, Qalitex is the dependable partner you need for:
- ISO 17025 accredited testing laboratory services
- FDA-compliant and USP AOAC validated analytical testing
- Microbiology, heavy metal, and shelf-life expertise
- Custom method development & regulatory compliance consultation
As liquid supplements gain a larger share of the global nutritional products market, investing in quality assurance with a certified lab like Qalitex ensures your brand’s reputation and consumer safety stand above the rest.
Get a quote today from Qalitex’s Irvine-based laboratory and boost your liquid dietary supplement’s market success:
Frequently Asked Questions (FAQs) About Liquid Dietary Supplements Market Testing and Qalitex Services
1. What makes Qalitex an ISO 17025 accredited testing laboratory for liquid dietary supplements?
Qalitex’s ISO 17025 accreditation means it meets international standards for technical competence and reliable testing results. This accreditation ensures liquid dietary supplement analyses are conducted using validated, reproducible methods essential for regulatory filings and product safety assurance. Learn more about Qalitex’s accreditation here.
2. How does Qalitex perform FDA compliant product testing on liquid dietary supplements?
Qalitex offers comprehensive chemical, microbiological, and heavy metal testing aligned with FDA regulations. The lab validates label claims and screens for contaminants, ensuring products meet US regulatory safety and efficacy requirements. Their testing protocols help brands avoid costly recalls and compliance issues. See their FDA compliant testing services for details.
3. Why are USP AOAC validated methods important for liquid supplement analysis?
USP and AOAC validated methods provide scientifically proven protocols that guarantee accuracy and consistency when quantifying active ingredients and contaminants in liquid supplements. Using these methods, Qalitex ensures data integrity for label claims and regulatory approvals. More about their method development and validation capabilities can be found here.
4. What core laboratory services does Qalitex offer for quality testing of liquid dietary supplements?
Qalitex delivers an extensive range of services tailored for liquid supplements, including microbiology testing, analytical chemistry testing, heavy metal analysis, shelf-life/stability studies, and custom method development and validation.
5. How can Qalitex help prove regulatory compliance for liquid dietary supplements sold on Amazon or in retailers?
Qalitex provides detailed testing reports and certificates of analysis (CoA) that verify product safety and label accuracy. These documents are often required by marketplaces like Amazon for compliance verification. Their CoA testing and regulatory compliance lab services support quick approval and ongoing compliance.
6. Why is heavy metal analysis critical for liquid dietary supplements, and how does Qalitex’s testing ensure safety?
Heavy metals like lead and arsenic can accumulate during raw material sourcing or manufacturing and pose serious health risks. Qalitex uses sensitive analytical techniques to detect these contaminants at trace levels, ensuring products meet strict regulatory limits and consumer safety standards. Visit their heavy metal testing service page for more info.
7. What shelf-life and stability study options does Qalitex provide for liquid dietary supplements?
Qalitex offers comprehensive shelf-life and real-time accelerated stability studies that simulate storage conditions to assess physical, chemical, and microbial stability of liquid dietary supplement products. These studies help brands determine accurate expiration dates and optimal packaging. Explore their stability testing services.
8. How can I get started with Qalitex for testing my liquid dietary supplements in Southern California?
You can request a quote online or contact their Irvine, CA laboratory directly to discuss your product’s testing requirements. Qalitex offers personalized laboratory testing strategies to meet timelines and budget while ensuring full regulatory compliance.
“`