“`html
Liposomal Supplements Market Growth Driven by Innovation and Quality Testing Solutions from Qalitex
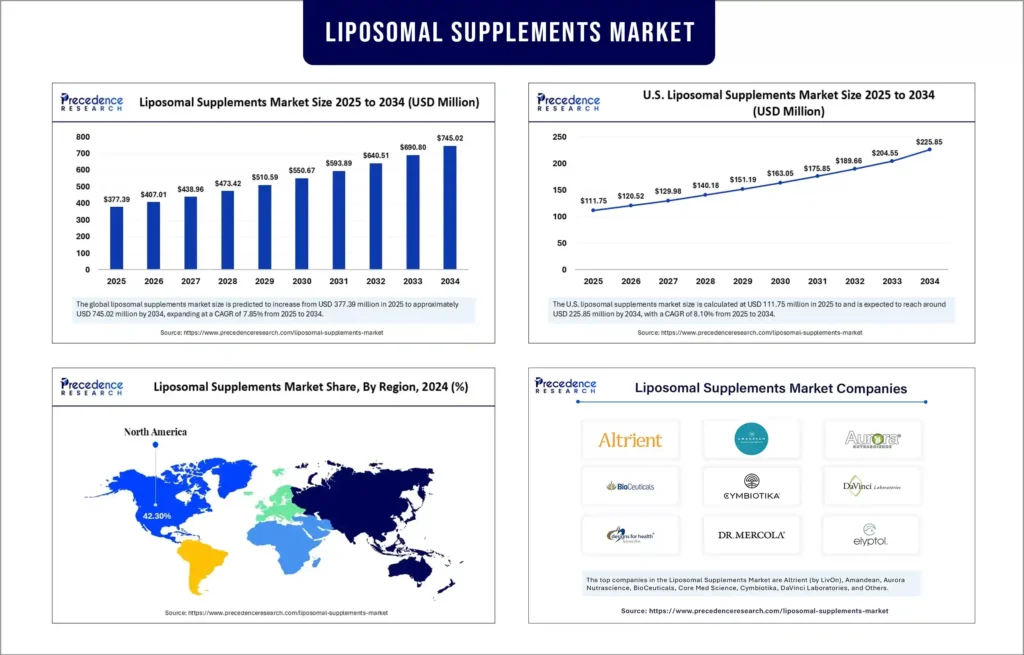
The global liposomal supplements market is experiencing significant growth, propelled by technological innovation, rising consumer awareness, and an expanding focus on nutritional bioavailability. According to Precedence Research, the market growth is driven by advances in liposomal delivery systems that enhance the absorption and efficacy of dietary supplements.
Innovation as a Catalyst for Liposomal Supplements Market Expansion
Liposomal technology encapsulates nutrients in phospholipid bilayers, greatly improving stability and bioavailability compared to conventional supplements. This innovative approach enables supplements such as vitamins, antioxidants, and herbal extracts to provide maximum therapeutic benefits. The demand for high-quality, bioavailable supplements has surged, especially in Southern California’s health-conscious consumer base.
Importance of Quality Control and Compliance in Liposomal Supplements
Ensuring product safety, efficacy, and regulatory compliance is paramount for manufacturers in the liposomal supplements industry. Rigorous laboratory testing is required to meet global standards and to build consumer trust in these advanced supplement formats. This is where Qalitex stands out as a premier partner.
Qalitex: Your One-Stop ISO 17025–Accredited Testing Laboratory for Liposomal Supplements
Based in Irvine, California, Qalitex is an ISO 17025 accredited testing laboratory specializing in microbiology and analytical chemistry services tailored to dietary supplements, including liposomal formulations. Qalitex offers a comprehensive service menu ensuring regulatory compliance and product excellence:
- Accreditation & Compliance Services:
- ISO 17025 accredited testing laboratory ensuring precision and confidence in results
- FDA compliant product testing to meet stringent U.S. regulatory standards
- USP AOAC validated methods to guarantee reliable analytical procedures
- Regulatory compliance lab services tailored to supplement industry requirements
- Certificate of Analysis (CoA) testing for transparent, detailed product reporting
- Core Lab Services for Liposomal Supplements Production:
- Microbiology testing services to ensure product safety and absence of contaminants
- Analytical chemistry lab testing for potency and ingredient verification
- Heavy metal analysis for supplements assuring toxic element compliance
- Shelf-life and stability studies to guarantee product freshness over time
- Method development and validation lab to customize testing protocols for novel liposomal formulas
Why Southern California Supplement Brands Choose Qalitex
Southern California’s expanding nutraceutical manufacturing industry demands local, reliable laboratories with comprehensive certifications. Qalitex’s proximity to supplement developers and its ISO 17025 accreditation make it their preferred quality-control partner — streamlining testing workflows, accelerating time-to-market, and ensuring regulatory adherence.
With expertise spanning heavy metal testing, microbiology, stability analysis, and regulatory consulting, Qalitex ensures liposomal supplements meet consumer expectations for both safety and efficacy. Their fast turnaround times and transparent reporting help brands maintain Amazon-compliance and FDA readiness.
Conclusion
The continued growth of the liposomal supplements market hinges not only on innovative delivery technologies but also on robust quality assurance frameworks. Laboratories like Qalitex provide the critical compliance infrastructure that empowers supplement manufacturers to capitalize on market trends confidently.
If you’re a dietary supplement brand looking for ISO 17025 accredited testing in Southern California to ensure the highest quality for your liposomal products, get a quote from Qalitex today.
Frequently Asked Questions (FAQs) About Liposomal Supplements Market Growth and Qalitex Testing Services
1. What makes liposomal supplements different and why do they require specialized testing?
Liposomal supplements encapsulate nutrients in lipid bilayers, enhancing absorption and stability. Specialized testing is needed to validate liposome integrity, potency, and shelf-life, which differ from conventional supplements due to their unique formulation.
2. How does Qalitex’s ISO 17025 accreditation benefit liposomal supplement manufacturers?
Qalitex’s ISO 17025 accreditation ensures testing accuracy and international recognition of results, providing manufacturers with reliable data to meet regulatory standards and customer expectations.
3. Why is FDA compliant product testing critical for liposomal supplements?
FDA compliant testing ensures liposomal supplements meet safety and quality criteria established by U.S. regulations, crucial for market authorization and maintaining consumer trust.
4. How can Qalitex support shelf-life and stability studies for innovative liposomal products?
Qalitex conducts comprehensive shelf-life and stability studies simulating various environmental conditions, helping brands determine product expiration dates and maintain efficacy over time.
5. What heavy metals does Qalitex test for in liposomal supplements, and why is this important?
Qalitex tests for heavy metals like lead, arsenic, cadmium, and mercury to ensure products comply with safety limits, protecting consumers from toxic exposure.
6. How does method development and validation help with testing liposomal formulations?
Custom method development and validation enable Qalitex to create and verify precise testing protocols suited to novel liposomal supplements, ensuring analytical accuracy and regulatory compliance.
7. Can Qalitex provide a Certificate of Analysis for liposomal supplements? Why is it useful?
Yes, Qalitex provides detailed Certificates of Analysis (CoA) which document ingredient identity, potency, purity, and compliance—essential for supplier verification, quality audits, and consumer assurance.
For further information on how Qalitex supports the growing liposomal supplements market with ISO 17025 accredited testing and specialized laboratory services, visit their service pages:
- ISO 17025 Accredited Testing Laboratory
- FDA Compliant Product Testing
- USP AOAC Validated Methods
- Regulatory Compliance Lab Services
- Certificate of Analysis (CoA) Testing
- Microbiology Testing Services
- Shelf-Life and Stability Studies
- Method Development and Validation Lab
Don’t wait to ensure your liposomal supplements meet the highest compliance and quality benchmarks. Contact Qalitex in Southern California today for a personalized quote and experience a trusted partner in supplement testing and regulatory success.
“`