“`html
India Dietary Supplements Market: Growth, Regulatory Challenges & Quality Testing Solutions by Qalitex
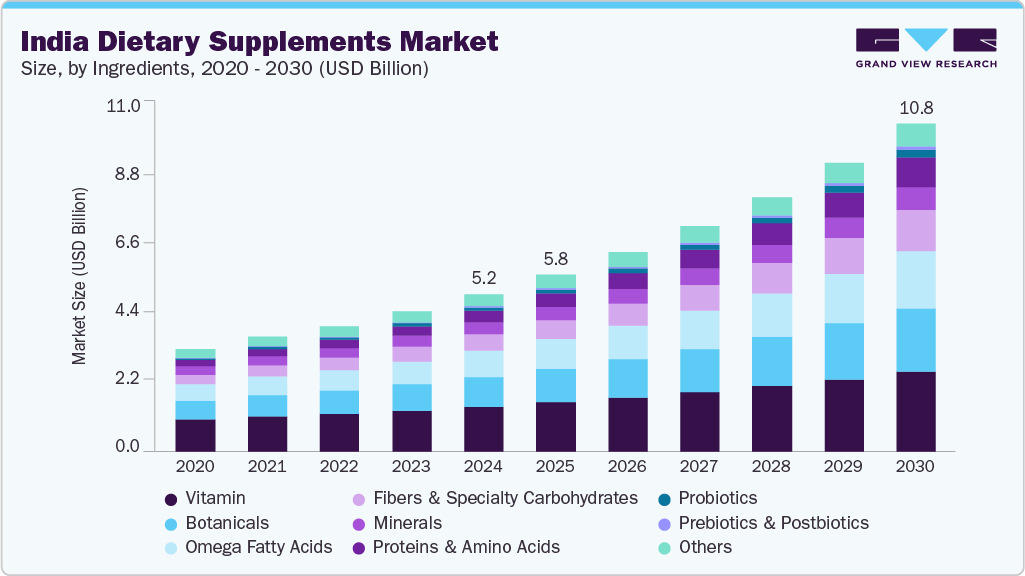
The India dietary supplements market is witnessing robust expansion owing to increasing health awareness, a surge in fitness consciousness, and a growing geriatric population emphasizing preventive healthcare. According to a detailed analysis by Grand View Research, this market is expected to experience significant CAGR throughout the coming decade, driven by enhanced consumer spending, demand for natural and herbal supplements, and evolving regulatory frameworks.
Market Drivers & Regulatory Landscape in India
India has witnessed a dynamic shift toward using supplements such as vitamins, minerals, herbal extracts, and sports nutrition products as part of daily wellness routines. However, as the market expands, regulatory oversight by agencies like FSSAI gets increasingly stringent, emphasizing stricter quality and safety benchmarks. This shift creates critical demand for advanced product testing to ensure compliance with national and international standards.
Importance of Compliance & Accreditation in Dietary Supplement Testing
To navigate the complexities of Indian regulatory requirements effectively, supplement manufacturers and exporters must leverage laboratories that offer ISO 17025 accredited testing and FDA compliant product testing. Such credentials guarantee that test results are reliable, reproducible, and globally recognized, facilitating smoother approvals and market entry.
Qalitex, located in Irvine, California, is a leading ISO 17025 accredited testing laboratory catering to dietary supplement brands looking to meet stringent quality control standards. Their services empower Indian brands and exporters to certify product safety and efficacy with confidence.
Qalitex’s Core Value Propositions for the India Dietary Supplements Market
Qalitex’s comprehensive testing portfolio aligns perfectly with the needs of dietary supplement businesses expanding to or exporting from India by offering:
- Accreditation & Compliance Services: Including FDA compliant product testing, USP AOAC validated methods, regulatory compliance lab services, and precise certificate of analysis (CoA) testing.
- Core Lab Analytical & Microbiological Services: Microbiology testing, analytical chemistry lab testing, heavy metal analysis for supplements, shelf-life and stability studies, and method development and validation tailored to dietary supplements.
Why Choose Qalitex for Dietary Supplements Testing?
With strict adherence to international quality standards, Qalitex serves as a one-stop quality-control partner for regulated consumer-product brands. Their expertise in comprehensive laboratory testing ensures that Indian dietary supplement producers can meet export protocols, reduce product recalls, and enhance consumer trust across global markets.
Key Testing Services for Dietary Supplements Relevant to India Market
1. ISO 17025 Accredited Microbiology Testing Services
Ensuring microbial safety is paramount. Qalitex’s accredited microbiology laboratory conducts thorough microbiological risk assessments to detect pathogens and spoilage organisms critical for FSSAI and international compliance. Learn more.
2. Heavy Metal and Contaminant Analysis
Heavy metals like lead, mercury, arsenic, and cadmium pose health hazards if not controlled. Qalitex’s precise heavy metal analysis for supplements assures products meet safety limits acceptable in India and other markets.
3. Shelf-Life and Stability Studies
Determining the product’s usable life under various storage conditions optimizes quality, potency, and consumer satisfaction. Qalitex offers customized shelf-life and stability studies for Indian market formulations.
4. Method Development and Validation
Qalitex aids supplement manufacturers in developing and validating reliable testing methods compliant with USP and AOAC standards, facilitating regulatory submissions and quality control.
Conclusion: Driving Success in the India Dietary Supplements Market with Qalitex Quality Testing
As India’s dietary supplements industry scales new heights, partnering with an ISO 17025 accredited and FDA compliant laboratory like Qalitex offers a competitive advantage — ensuring products not only comply with local regulations but also appeal to discerning global consumers.
Located in Southern California, Qalitex is strategically positioned to serve Indian supplement exporters and domestic brands seeking rigorous quality assurance and compliance testing. Request a quote today to elevate your supplement quality: Get Your Testing Quote
Frequently Asked Questions (FAQs) about India Dietary Supplements Market & Qalitex Testing Services
1. What makes Qalitex an ideal ISO 17025 accredited testing laboratory for dietary supplements targeted at the Indian market?
Qalitex’s ISO 17025 accreditation ensures globally recognized technical competence and quality management, critical for meeting India’s FSSAI and international standards. Their advanced microbiology, heavy metal analyses, and method validations offer trusted data to supplement manufacturers.
2. How does Qalitex help dietary supplement producers in India achieve FDA compliant product testing?
Qalitex’s FDA compliant product testing features validated chemical and microbiological assays aligned with FDA guidelines, supporting Indian companies that export supplements to the U.S. or need compliance aligned with FDA food additive and supplement requirements.
3. Why is heavy metal analysis important for dietary supplements in India, and how does Qalitex perform this testing?
Heavy metals pose health risks and have stringent allowable limits in India. Qalitex employs sensitive analytical chemistry methods to quantify trace metals ensuring supplements comply with the limits prescribed by FSSAI and global regulators.
4. What role do shelf-life and stability studies play for supplements marketed in India, and can Qalitex assist?
Shelf-life studies validate product potency and safety over time under typical storage. Qalitex provides tailored stability testing, enabling Indian brands to assign accurate expiry dating and ensure consistent quality post-launch.
5. How can Qalitex’s method development and validation services benefit Indian dietary supplement manufacturers?
Qalitex helps develop proprietary or adapted analytical methods that conform to USP and AOAC validated standards, giving manufacturers a competitive edge with GMP-compliant quality testing processes in India.
6. Where is Qalitex located, and can they provide services to supplement companies in Southern California and India?
Qalitex is based in Irvine, California, providing a convenient West Coast hub for Indian exporters and local companies in Southern California seeking reliable accreditation-driven lab services for dietary supplements.
“`