“`html
Fatty Acid Supplements Market: Driving Quality, Compliance & Innovation with Qalitex’s Expert Lab Testing Services in Southern California
Published: June 2024
Overview of the Global Fatty Acid Supplements Market
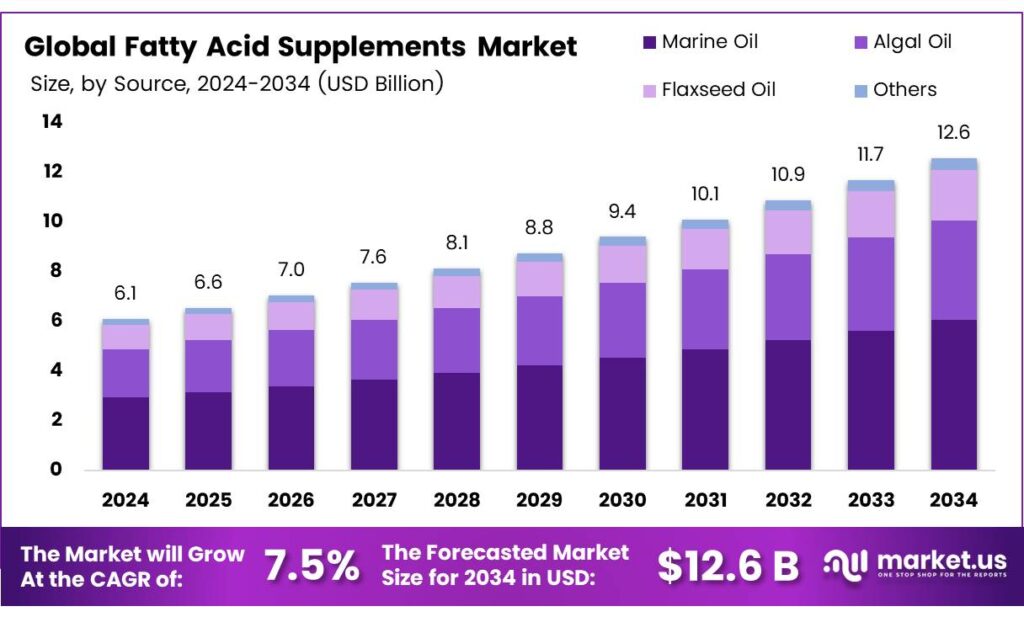
The Fatty Acid Supplements Market continues its robust growth trajectory, propelled by rising consumer awareness about health and wellness, increasing demand for omega-3 and other essential fatty acids, and expanding applications in dietary supplements. According to Market.US, this market is projected to witness significant CAGR through 2028, with innovation in product formulations and rising regulatory scrutiny driving demand for high-quality, tested supplements.
In this highly regulated industry, ensuring product safety, quality, and regulatory compliance is paramount, positioning specialized laboratories as critical partners for manufacturers. Southern California-based Qalitex excels as an ISO 17025–accredited microbiology and analytical chemistry laboratory delivering comprehensive testing services for fatty acid supplements along with dietary and cosmetic products.
Qalitex: Your One-Stop Lab Partner for Fatty Acid Supplements Testing & Compliance
Qalitex is strategically located in Irvine, California, offering advanced ISO 17025 accredited testing laboratory services essential for fatty acid supplement producers seeking regulatory compliance across multiple markets. Its lab testing capabilities encompass:
- Analytical chemistry lab testing specialized in fatty acid quantification and compositional analysis.
- Heavy metal analysis for supplements ensuring products meet stringent FDA and international contaminant limits.
- Shelf-life and stability studies to optimize product efficacy and guarantee label claims.
- Microbiology testing services to certify absence of harmful microbes.
- Method development and validation labs for customized testing protocols compliant with USP AOAC validated methods.
By integrating these services, Qalitex provides fatty acid supplement manufacturers with a fully traceable, comprehensive quality assurance program—from raw material verification to finished product testing and regulatory reporting.
Accreditation & Compliance Services Tailored for Fatty Acid Supplements
Qalitex’s reputation as a top-tier testing partner is anchored in its strict adherence to international standards and regulatory requirements:
- ISO 17025 Accredited Testing Laboratory: Demonstrates technical competence and reliability in testing fatty acid supplements. Learn more.
- FDA Compliant Product Testing: Ensures testing methodologies and results meet FDA guidelines, accelerating market entry. Discover FDA compliant testing.
- USP AOAC Validated Methods: Validated analytical protocols support standardized fatty acid analyses and global acceptance. Explore validated methods.
- Regulatory Compliance Lab Services: Addresses compliance reporting, certificate of analysis (CoA) testing, and risk management for supplements. Full compliance services here.
- Certificate of Analysis (CoA) Testing: Provides verification documents essential for distributor and retailer trust. Learn about CoA testing.
These accreditation and compliance credentials make Qalitex an ideal laboratory partner to meet the stringent quality expectations of the fatty acid supplements market and retailer platforms like Amazon.
Core Lab Services Driving Quality Control in Fatty Acid Supplements
In addition to accreditation, Qalitex’s suite of core laboratory test services ensures every batch of fatty acid supplements meets label claims and safety standards:
- Microbiology Testing Services: Detection and quantification of microbial contaminants to prevent spoilage or health risks. View services.
- Analytical Chemistry Lab Testing: Comprehensive assays to determine fatty acid profiles, potency, and purity. Details here.
- Heavy Metal Analysis for Supplements: Screening for arsenic, lead, cadmium, mercury, and other heavy metals. Heavy metal testing explained.
- Shelf-Life and Stability Studies: Evaluates product integrity over time under various conditions to optimize formulation and packaging. Learn more.
- Method Development and Validation Lab: Customized testing protocols tailored to novel fatty acid supplement ingredients or delivery forms. Explore method services.
These core laboratory capabilities are instrumental in helping Southern California’s dietary supplement brands maintain competitive advantage by ensuring rigorous in-house and third-party product verification.
Why Southern California Fatty Acid Supplement Manufacturers Choose Qalitex
Located in Irvine, CA, Qalitex offers unparalleled access for Southern California-based manufacturers seeking rapid turnaround and personalized service that large national labs often cannot provide. Key benefits include:
- End-to-End Compliance Support: From raw material testing to finished product CoA generation including Amazon-compliance testing requirements.
- Cutting-Edge Analytical Technologies: State-of-the-art instrumentation ensures precise quantitation of complex fatty acid matrices.
- Experienced Scientific Team: Expertise in microbiology, analytical chemistry, and regulatory affairs specific to dietary supplements.
- Client-Centric Service Model: Customizable testing packages and consulting to meet unique product and regulatory needs.
- Local Presence with Global Standards: Combining Southern California convenience with international testing standards such as USP and AOAC.
By partnering with Qalitex, fatty acid supplement brands streamline their quality control process and accelerate market readiness with confidence.
Frequently Asked Questions (FAQs) about Fatty Acid Supplements Market Testing & Qalitex Laboratory Services
- What makes Qalitex an ideal ISO 17025 accredited testing laboratory for fatty acid supplements?
- Qalitex offers ISO 17025 accreditation, which assures that its testing procedures for fatty acid supplements meet internationally recognized quality and technical standards, ensuring reliable and legally defensible data.
- How does Qalitex ensure FDA compliant product testing for fatty acid supplements?
- Qalitex employs FDA guideline-aligned analytical protocols, including heavy metal screens and microbiological testing, to ensure fatty acid supplement products comply with FDA safety and purity requirements.
- Why are USP AOAC validated methods important for testing fatty acid supplements?
- USP AOAC validated methods provide standardized, scientifically recognized testing protocols that improve the accuracy and acceptance of fatty acid composition results in regulatory and commercial environments.
- What is involved in Qalitex’s heavy metal analysis for fatty acid supplements?
- Qalitex tests supplement samples for toxic metals like lead, arsenic, cadmium, and mercury, utilizing ICP-MS and other advanced techniques to confirm compliance with safety thresholds.
- How does Qalitex conduct shelf-life and stability studies for fatty acid supplements?
- Through controlled environmental testing over time, Qalitex measures fatty acid degradation, potency retention, and microbiological stability to establish accurate shelf-life dating.
- Can Qalitex develop customized testing methods for novel fatty acid supplement formulations?
- Yes, Qalitex offers method development and validation services tailored to unique ingredient profiles and delivery forms, ensuring precise and validated analysis.
- How can Southern California dietary supplement brands get a quote for fatty acid supplement testing?
- Local manufacturers can request a personalized testing quote from Qalitex online or by phone, receiving expert consultation tailored to their testing needs and timelines.
“`